Films of Reduced Graphene Oxide-Ni(OH)2 Nanowall Networks for Oxygen Evolution and Supercapacitor Applications
K. Bramhaiah, C. Alex, V. N. Singh and Neena S. John, ChemistrySelect 2019, 4, 2519–2528
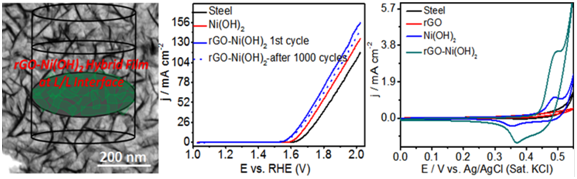
Free-standing films of reduced graphene oxide (rGO) based Ni(OH)2 nanowall structures are generated at a liquid/liquid interface involving in situ reaction and self-assembly. The nanowall network of Ni(OH)2 sheets anchored on rGO layers with 10–15 nm wall thickness and ordered voids of average size 100 nm can serve as excellent candidates for catalyzing electrochemical oxygen evolution reaction (OER) as they expose maximum edge sites of Ni(OH)2 and allow diffusion and penetration of electrolytes into voids enhancing the contact area of the electrolyte/electrocatalyst interface. The unique morphology of the hybrid films exhibited high electrochemical surface area and low charge transfer resistance. Accordingly, rGO-Ni(OH)2 hybrid films display excellent catalytic activity and high cycling stability in alkaline solutions giving a current density of 10 mA/cm2 at an overpotential of 378 mV with a Tafel slope of 56 mV/dec. The hybrid films also exhibited a high specific and areal capacitance of 1402 F/g and 98.12 mF/cm2 at a scan rate of 5 mV/s. OER activity and capacitance of the hybrid rGO- Ni(OH)2 films are higher compared to that of bare Ni(OH)2 film and is attributed to the synergic effect between the rGO layer and the ordered interconnected nanowall Ni(OH)2 nanostructures. The primary advantage of these hybrid films is that they can be collected on the desired substrate for ready use as electrodes without the use of any external binders.
