Influence of Iodine doping on the Structure and Properties of Metal-Phthalocyanine Thin Films
K. Priya Madhuri, Pralay K. Santra, F. Bertram and Neena S. John, Phys. Chem. Chem. Phys., 2019, 21, 22955-22965;
K. Priya Madhuri, Neena S John, S Angappane, P. K Santra, F. Bertram, J. Phys. Chem. C, 2018, 122, 49.
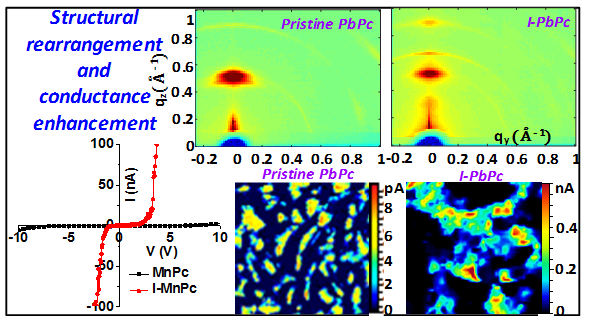
Doping with halide ions is a popular method to alter the electrical and magnetic properties of metal phthalocyanines (MPcs). Oxidative doping in planar and nonplanar MPcs also causes structural rearrangements in molecular packing and dependent physical properties. Two-dimensional grazing incidence X-ray diffraction (2D-GIXRD) studies of pristine and iodine doped MPcs reveal that the ordered molecular packing undergoes rearrangement to give to differently oriented crystallites with interstitial iodide species. The ferromagnetic interactions between the macrocycles of pristine MnPc molecules seem to get suppressed while the conductance increases drastically, revealed by conducting-atomic force microscopy (C-AFM). The oxidative doping of MnPc molecules with iodine enhances the p-type conductivity and provides conductive iodine percolation pathways, thereby enhancing the conductance. 2D-GIXRD of pristine and iodine doped nonplanar PbPc films reveal similar structural changes and the nanoscale conductance mapping in iodine doped PbPc films using C-AFM reveal interconnected iodine percolation pathways which are in contrast to the isolated conducting domains in the pristine films. It is also shown that the competitive doping in PbPc films enhances the sensitivity towards analyte sensing by studying the effect of ammonia on the conductance of iodine doped PbPc films.