Some days ago, some of my friends and I went to a hill viewpoint by trekking. Before we started from our room, one of my friends was applying sunscreen lotion on her skin and she suggested that I also use that sunscreen to avoid tan. Then she tried to convince me to use the sunscreen lotion of that particular company by mentioning the “SPF”. But when I enquired about the meaning of SPF and how much sunscreen lotion can protect our skin, she could not able to explain it. After that incident, I felt a lack of awareness about the ozone layer and its importance among us. More importantly, what small habits could we adopt daily as responsible human beings of this planet to protect the ozone layer? At that moment, I gave long preaching to my friend, which I have summarized in this blog to spread the awareness at a larger scale.
Let me start with the term “SPF” where it all started. SPF stands for Sun Protection Factor. It illustrates how much the usage of a sunscreen lotion reduces the risk of skin damage from UV rays. The factor is defined as the ratio of “time taken to cause skin reddening when sunscreen lotion applied (2mg on 1 sq. cm. of skin surface)” to “time taken to cause skin reddening when no sunscreen lotion applied”. In principle, if it takes 10 minutes for unprotected skin to get red under given UV conditions, a sunscreen lotion with SPF 15 would prevent this for 150 minutes.
I will briefly discuss ultraviolet (UV) rays and their components in the solar light spectrum. UVA, UVB, and UVC are the three kinds of ultraviolet radiation emitted by the sun. UV radiation can harm your skin in any form, but each type has a different effect. UVA having wavelength of range 400 nm – 314 nm, create wrinkles, “sun spots,” and other signs of premature aging since they account for 95 percent of the radiation that reaches the earth’s surface. UVA is minutely associated with skin cancer development. Whereas UVB (314 nm – 280nm) rays, which impact the top layer of the skin, are mainly responsible for skin sunburns and significantly contribute to causing skin cancer. Sunscreen lotions mainly protect our skin from UVB radiation, and the protection level depends on its SPF value. But still, no sunscreen can protect our skin from UV rays 100%. One sunscreen contains four types of ingredients – 20 % active ingredients, 55 % formulation stabilizers, 23 % sensory enhancers, and 2 % added extras. Among them, the active ingredients have the role of protecting our skin from UV radiations of the sun by either absorbing or deflecting from the skin. Depending on the active ingredients, sunscreens are classified into two types – physical sunscreens and chemical sunscreens. Physical sunscreens work by deflecting sunlight from the skin through ingredients like titanium dioxide and zinc oxide. In contrast, chemical sunscreens work through carbon-containing elements like octinoxate, oxybenzone, octocrylene, avobenzone, homosalate, octisalate by absorbing sunlight from skin and radiating as heat. The formulation stabilizers help to protect the life of active ingredients by dissolving them in proper solvents; sensory enhancers give the feel of the sunscreen on skin through fragrance or moisturizing properties; the added extras stand to provide a unique characteristic to a particular sunscreen in industry and some of them help to protect skin from pollution.
The most harmful UV radiation is UV-C (280-100nm) never reaches earth because it gets absorbed by our natural sunscreen protector “ozone layer”. We all have read about this natural sunscreen protector in our school textbooks. This layer contains the higher concentration of Ozone (O3), found in the stratosphere at about 15 to 35 kilometers from the earth’s surface is known as the Ozone layer or Ozonosphere. It helps to preserve life on earth by resisting the incident of harmful UV radiation from the sun. We all could imagine the day when our natural sunscreen “ozone layer” gets disappear. It will be doomsday for humanity. But we don’t bother much about it; instead, we prefer our daily life comfort over it.
In the early 1970s, scientists first realized the effect of human activity on reducing ozone concentration in the atmosphere. They found the CFCs (chlorofluorocarbons) as potent ozone depleting substances originating from uses of refrigerator and different kinds of sprays since 1930s. Ozone depletion is the continuous thinning process of the ozone layer at stratosphere level by generating chemicals known as Ozone Depleting Substances (ODS) like chlorofluorocarbon (CFC), carbon tetrachloride (CCl4), hydrochlorofluorocarbon (HCFC), methyl chloroform – mainly the halocarbons containing chlorine or bromine or fluorine radicals by human activities. The halocarbons like CFC, CCl4, have the source in the packaging or refrigeration industries. These ODSs can sustain in the environment for almost 100 years and one molecule of chlorine can break down thousands of ozone molecules.
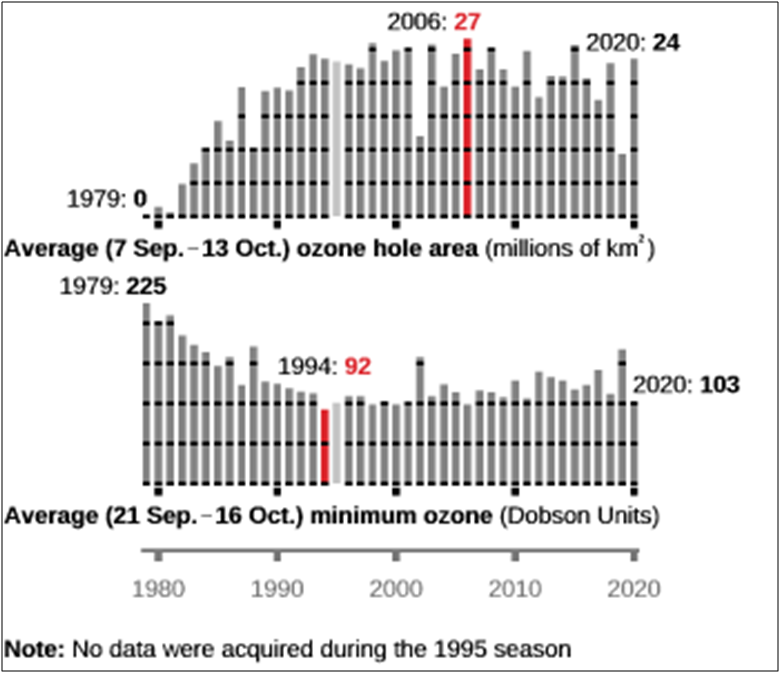
In the 1980s, the significant depletion of our natural sunscreen was noticed in the polar region, especially every spring. The severe ozone depletion that occurred over Antarctica was named as Ozone hole. Till 1978, scientists did not observe the concentration of ozone below 220 Dobson units in the atmosphere. The minimum concentrations of the ozone were 194, 190, 173, 154 and 124 in the years 1979, 1980, 1982, 1983 and 1985, respectively. The maximum reduction in concentration occurred in 1994 with 73 Dobson units.
To stop the enhancement of ozone depletion, World Ozone Day is celebrated every year on 16th September. On this day, Montreal Protocol on substances that deplete the Ozone layer and its amendments were signed in 1987.This international treaty strictly bans the emission of 99% of ODS since 1987 and as a result, now the recovery can be observed in the ozone layer. If the Montreal Protocol had not been signed, ozone depletion would have been affected both humans and plants. The UV radiation would increase the skin problems which might be carried over to next generations. For this harmful radiation, plants also can’t absorb more CO2 like usual, so it indirectly would increase the earth’s overall temperature and could be responsible for global warming. Then whatever SPF one sunscreen would have possessed, it would not protect our skin from this level of high exposure to UV radiation.
If I come to our activity to protect our natural sunscreen, then I must say that we have the primary responsibility to save the earth by preserving the ozone layer. We can do it in several ways, like using the air conditioner containing R32 gas, using eco-friendly insulating materials, reducing the use of cosmetics, aerosols, hair sprays, using bubble wraps instead of thermocol. Finally, the main important work is to plant trees as much as possible. Our small steps can lead our life to a better future.
After listening to all my preaching, my friend was about to spray her perfume but stopped spraying and she also promised me that she would reduce using perfume or any kind of body spray. I smiled and became happy by observing the effect of my words. Thus, if everyone in the world starts taking such small steps in order to protect the existence of our natural sunscreen from today, we can definitely gift a better world to our next generations.
Ms Pritha Dutta
Junior Research Fellow
Group of Dr Ashutosh Singh
CeNS, Bengaluru
References
- https://www.britannica.com/science/ozone-depletion
- https://www.un.org/en/observances/ozone-day
- https://www.researchgate.net/publication/344283614_Ozone_day_article
- https://ozonewatch.gsfc.nasa.gov/
- https://earthobservatory.nasa.gov/world-of-change/Ozone
- https://www.aad.org/public/everyday-care/sun-protection/sunscreen-patients/is-sunscreen-safe
- https://www.chemicalsafetyfacts.org/chemistry-context/sunscreen-summertime-safety/
- https://cen.acs.org/business/consumer-products/What-in-sunscreen-and-how-does-it-protect-your-skin-from-the-sun-rays/99/i27

Good read Pritha! Thanks for the information!
You have presented the real problems in a very simple and beautiful way.👌
Wonderful! Well written, Pritha! Useful information..
so nicely written.
Awsome Pritha… U r just amazing… Nicely briefed… Its very useful… Thank you for the awareness.
Gre8…. Useful info. 👍🏻👍🏻
A well documented informative writing Ms. Pritha.
Nicely written Pritha!
Very well written Pritha ma’am…and thanks for making us aware..
Thank you all.
Excellent writing and very informative.